Antigenic Proteins of excretory and secretory products purified from Cotylophoron cotylophorum.
Keywords:
<i>Cotylophoron</i>, proteins, bovines, antigensAbstract
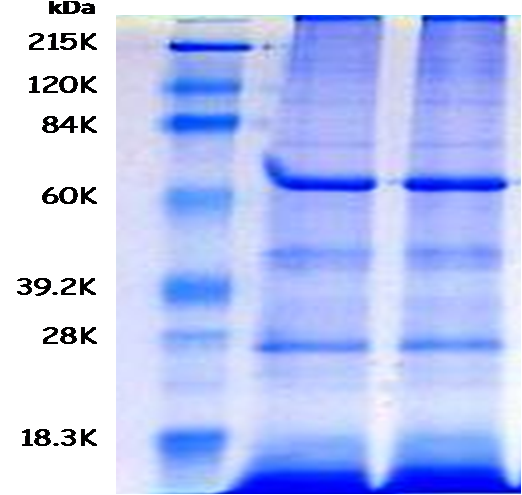
To diagnose the trematode Cotylophoron cotylophorum by the sedimentation and sieve technique has low efficiency when the parasite burden is low, because such technique has low sensitivity to diagnose the trematode eggs in the fecal samples. The main objective of this research was to purify some proteins from the secretory and excretory products of adult stages of C. cotylophorum with the aim of assessing its antigenicity by immunoelectrotransferency (Western Blot technique), next those proteins were used as antigens in an enzyme link immune assay (ELISA). First, 1,200 adult C. cotylophorum, collected directly from bovine´s rumen slaughtered at the local Matadero Industrial Centroccidental (MINCO), were incubated during 16 hours in Minimun Essential Eagle (MEM), and antigenic proteins were obtained from this MEM. Next, proteins were purified and concentrated by ultracentrifugation. An electrophoresis technique (SDS-PAGE) and a Western Blot were carried out to identify the antigenic proteins using an hyperimmune serum obtained from previously immunized rabbits with the purified proteins isolated from the excretory and secretory products of C. cotylophorum. Nine (9) protein bands were identified with molecular weights of: 17, 24, 43, 56, 62, 76, 83, 105, and 121 KDa, and three of these bands (62, 76, and 105 KDa) were recognized by the serum from four (4) bovines which had previously been diagnosed as positives to C. cotylophorum by coprologic tests. The protein with 76 KDa was the most reactive. Finally, these purified antigens may be used to develop immunoenzymatic assays with greater sensitivity and specificity, which would be very helpful tests for the diagnostic and epidemiologic study of C. cotylophorum in Venezuela.
Downloads
References
[2] Marquez D. Resistencia a los antihelmínticos: origen, desarrollo y control. Rev CORPOICA 2003; 4(1): 55-71.
[3] García I, Muñoz B, Aguirre A, Polo I, García A, Refoyo P. Manual de laboratorio de Parasitología. Introducción a los Helmintos. Trematodos. Reduca
(Biología). Serie Parasitologpia 2008; 1(1): 67-93.
[4] Sanabria R, Romero J. Review and update of paramphistomosis. Helminthologia 2008; 45(2): 1-5.
[5] Khatoon N, Mujib B, Mirza S. Histological changes in the liver of buffaloes by digenetic tremátode Paramphistomun cervi. Pakistan J Biol Sc 2003; 6(17): 1540-1543.
[6] Muro A, Ramajo M. Paranfistomosis. En: Cordero del Campillo M, Rojo FA. (Eds). Parasitología Veterinaria 2002; 3ª Edicion, Madrid: McGraw-Hill Interamericana. p. 225-228.
[7] Singh D, Lakra P. Pathologic changes in naturally occurring Cotylophoron cotylophorum infection in cattle. Am J Vet Res 1971; 32: 659-663.
[8] Benedek L. Examination of liver fluke eggs with sedimentation technique. Allatorov Lapak, 1946; 66:139-140.
[9] Ibarra F, Montenegro N, Vera Y, Boulard C, Quiroz H, Bautista C, Vázquez C. DIG-ELISA: Estandarización y evaluación serodiagnóstica en fasciolosis bovina experimental y natural. Vet Méx 1997; 28(1): 7-12.
[10] Torrel P. Detección de coproantígenos de Fasciola hepatica en ovinos y bovinos mediante un método de ELISA. Rev Invest Pec IVITA. 1997; 6(1): 74-78.
[11] Hillyer G, De Galanes S, Rodríguez J, De Lagrava, M, Ramírez S, Bryan R. Use of the Falcon assay screening test-enzyme-linked immunosorbent assay (FAST ELISA) and the Enzyme-linked Immunotransfer Blot (EITB) to determine the prevalence of human fascioliasis in the Bolivian Altiplano. Am J Trop Med Hyg 1992; 46: 603-609.
[12] Cordova M, Reategui L, Espinoza JR. Immunodiagnosis of human fascioliasis with Fasciola hepatica cysteine proteinases. Trans Royal Soc Trop Med Hyg 1999; 93(1): 54-57.
[13] Escalante H, Davelois K, Ortiz P, Rodríguez H, Díaz E, Jara C. Estandarización de la técnica de Western Blot para el diagnóstico de la Fasciolosis humana utilizando antígenos de excreción-secreción de Fasciola hepatica. Rev Per Med Exp Salud Públ 2011; 28(3): 454-61.
[14] Higuita E, Muñoz D, Marín M, Velásquez L. Aproximación al diagnóstico serológico de la infección causada por Cotylophoron sp en Colombia. Rev Colom Cs Pec 2007; 20: 4-6.
[15] Dorchies P, Lacroux C, Levasseur G, Alzieu J. La paramphistomose bovine Bull. Group Tech. Vét 2002; 13:13-16.
[16] Alarcón E, Velásquez L. Descripción morfológica de Cotylophoron cotylophorum (Digenea: Paramphistomidae) hallado en bovinos de Rionegro, Antioquia, Colombia. Rev Colomb Cs Pec 2009; 22:168-177.
[17] Sarimehmtoglu, H. Application of Western blotting for the immunodiagnosis of Fasciola hepatica in cattle using excretory/secretory antigens. Turkish J Vet Anim Sci 2002; 26: 1061-1065.
[18] Fine J. The biuret method of estimating albumin and globulin in serum and urine. Bioch J 1935; 29(3): 799-803.
[19] Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970; 227:680-685.
[20] FONDO NACIONAL DE CIENCIA, TECNOLOGÍA E INNOVACIÓN (FONACIT). Norma para utilización de animales en investigación. En: Código de Bioética y Bioseguridad. Capítulo 3. Tercera edición. Caracas, Venezuela, 2009; p. 33-35.
[21] Tsang V, Peralta J, Simons A. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Meth Enzymol 1983; 92: 377-391.
[22] Ruiz M, Arriaga C, Bautista C, Morilla A. Fasciola hepatica: characterization of somatic and excretory-secretory antigens of adult flukes recognized by infected sheep. Rev Latinam Microbiol 1993; 35(3): 301-307.
[23] Sampaio M, Da Costa J, Da Costa A, Pires M, Lopes S, Castro A, Monjour L. Antigenic components of excretory-secretory products of adult Fasciola hepatica recognized in human infections. Am J Trop Med Hyg 1996; 54(2): 146-148.
[24] Colmenares C, Méndez L, Díaz-Bello Z, Alarcón de Noya B. Antígeno excreción-secreción de Fasciola hepatica: ultrafiltración y aplicación en inmunodiagnóstico. Acta Bioquími Clín Latinam 2007; 41 (2): 259-266.
[25] Espino, A., Borges, A., Duménigo, B. Coproantígenos de Fasciola hepatica de posible utilidad en el diagnóstico de la fascioliasis. Pan Ame J Public Health 2000; 7(4): 225-231.
[26] Hillyer GV, Soler de Galanes M, Delgado Azañero E. Immune diagnosis of human fasciolosis in children from Cajamarca, Peru. Parasitol al Día 2001; 25 (3-4): 21-25.
[27] Diaz A, Li-Elias O, Otero O, Garcia C, Espino AM. Identificación, mediante Western blot, de inmunógenos de Fasciola hepatica, reconocidos por los sueros de ratas infectadas experimentalmente. Rev Cub Med Trop 1998; 50:12-17.
[28] Miranda, M., García Z. Aislamiento e identificación in situ de antígenos de Fasciola hepatica. Vet Méx 1994; 25(3): 267-271.
[29] Knobloch J. Human fascioliasis in Cajamarca/Perú. II. Humoral antibody response and antigenemia. Trop Med Parasitol 1985; 36(2): 91-93.
[30] Cornejo H, Oblitas F, Cruzado S, Quispe W. Evaluación de una prueba de ELISA con antígeno metabólico de Fasciola hepatica para el diagnóstico de fasciolosis humana en Cajamarca, Perú. Rev Per Med Experim Salud Públic 2010; 27(4): 569-574.
[31] Hanna R. Fasciola hepatica: an imunofluorescent study of antigenic changes in the tegument during development in the rat and the sheep. Experm Parasitol 1980a; 50: 155-170.
[32] Hanna R. Fasciola hepatica: glycocalyx replacement in the juvenile as a possible mechanism for the protection against host immunity. Experim Parasitol 1980b; 50:103-114.
[33] Fredes F, Alarcón J, Ilabaca P, Alcaíno H. Evaluación diagnóstica de dos proteínas purificadas de Fasciola hepatica mediante ELISA en fasciolosis ovina. Parasitol Latinam 2003; 58:148-151.
[34] Gomes, Y.; Pereira, V.; Nakazawa, V.; Rosa, D.; Barros, M.; Ferreira, A.; Silva, E., Ogatta, S.; Krieger, M. y Goldenberg, S. Serodiagnosis of chronic Chagas infection by using EIE-recombinant-Chagas-Biomanguinhos kit. Mem Inst Oswaldo Cruz 2001; 96:497-501.
[35] Nakazawa M, Rosa D, Pereira V, Moura MO, Furtado VC, Souza WV, Neves MD, Barros DS, Abath FGC and Gomes YM. Excretory-secretory antigens of Trypanosoma cruzi are potentially useful for serodiagnosis of chronic Chagas Disease, Clin Diag Lab Immunol 2001; 8(5): 1024-1027.
[36] Santarém N, Silvestre R, Tavares J, Silva M, Cabral S, Maciel J and Cordeiro-da-Silva. 2007. Immune response regulation by Leishmania secreted and nonsecreted antigens. J Biomed Biotechnol 2007; 85154. http://doi.org/10.1155/2007/85154
[37] De Almeida MA, Ferreira MB, Planchart S, Terashima A, Maco V, Marcos L, Gotuzzo G, Sánchez E, Náquira C, Scorza JV and Incani RN. Preliminary antigenic characterisation of an adult worm vomit preparation of Fasciola hepatica by infected human sera. Rev Instit Med Trop São Paulo 2007; 49(1): 31-35.
[38] Barbosa PR, Ferreira AW. Avidity of IgG antibodies against excreted/secreted antigens of Toxoplasma gondii: immunological marker for acute recent toxoplasmosis. Rev Socied Brasil Med Trop 2008; 41(2): 142–147.
[39] Marcilla, JE De la Rubia, J. Sotillo, D. Bernal, C. Carmona, Z. Villavicencio, D. Acosta, J. Tort, FJ. Bornay, JG. Esteban, and R. Toledo. Leucine Aminopeptidase Is an Immunodominant Antigen of Fasciola hepatica Excretory and Secretory Products in Human Infections. ClinVacc Immunol 2008; 15(1): 95-100.
Published
How to Cite
Issue
Section
Gaceta de Ciencias Veterinarias se apega al modelo Open Access, por ello no se exige suscripción, registro o tarifa de acceso a los usuarios o instituciones. Los usuarios pueden leer, descargar, copiar, distribuir, imprimir y compartir los textos completos inmediatamente después de publicados, se exige no hacer uso comercial de las publicaciones. Para la reproducción parcial o total de los trabajos o contenidos publicados, se exige reconocer los derechos intelectuales de los autores y además, hacer referencia a esta revista. La publicación de artículos se hace sin cargo para los autores. Los trabajos pueden consultarse y descargarse libremente, y de manera gratuita, en extenso en versión digital, desde su enlace Web institucional. Los textos publicados son propiedad intelectual de sus autores. Las ideas, opiniones y conceptos expuestos en los trabajos publicados en la revista representan la opinión de sus autores, por lo tanto, son estos los responsables exclusivos de los mismos.


