Study of hepatic damage of a high dose of Oxytetracycline in pregnant female mice (Mus musculus).
Keywords:
Oxytetracycline, hepatic damage, pregnancyAbstract
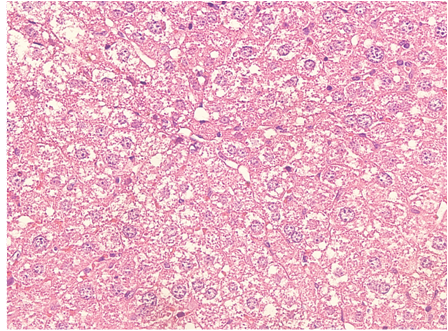
Among the antibiotics that can cause fatty liver (HG) is oxytetracycline (OT). This study aimed to determine the hepatic damage of a high dose of OT in pregnant female mice and its association with oxidative stress (OE). 30 NMRI female mice distributed in three groups of 10 animals: Day 0 not pregnant, to Parturition, maintained until delivery, to Weaning, maintained until weaning. In all, half was injected i.p. with a single dose of 100 mg/kg OT, experimental group. The results are the mean±SE, the statistical significance was obtained by Student´s t-test. HG was evaluated by histology and by the concentration of hepatic triglycerides. As OE parameters, the hepatic concentration of conjugated dienes (DC) and MDA was determined by spectrophotometry. Liver function was assessed by the plasma activity of the aminotransferases ALT and AST by commercial kits. The results showed that a high dose of OT, at 24 hours in non-pregnant female mice (Day 0 group) elevated the hepatic triglycerides, accompanied by hepatoesteatosis and increased plasma ALT and AST. OE was found in them, evidenced by high concentration of DC and MDA. At delivery, the experimental pregnant females did not present any modification of the parameters described in the Day 0 group, evidencing that at 21 days after the administration of a high OT dose, the effect of the anibiotic was not maintained, a similar result was obtained in the females at the time of weaning.
Downloads
References
[2] Armengol R, Fraile L. Comparison of two treatment strategies for cows with metritis in high-risk lactating dairy cows. Theriogenology 2015; 83(8):1344-1351.
[3]. De Briyne N; Atkinson J; L. Pokludová L; Borriello SP. Antibiotics used most commonly to treat animals in Europe.Vet Record 2014. 175(13): 327-335.
[4] Labbe G, Fromenty B, Fréneaux E, Morzelle V, Lettéron P, Berson A, and Pessayre, D. Effects of various tetra-cycline derivatives on in vitro and in vivo ß-oxidation of fatty acids, egress of triglycerides from the liver, accumulation of hepatic triglycerides, and mortality in mice. Biochem Pharmacol 1991; 41: 638-641.
[5] Ruiz de Rivero M, Mendoza C, López-Ortega AA. Estrés oxidativo en el hígado graso inducido por oxitetraciclina en ratones hembras. Gaceta Cs. Vet 2009; 14(2): 80-86.
[6] Hautekeete M, Degott C, Benhamou J. Microvesicular steatosis of the liver. Acta Clin Bleg 1990; 45(5): 311-326.
[7] Duarte J, Díaz S, Lee VE, Castro J, Velásquez V. Hígado graso agudo del embarazo: experiencia de 8 años. Med Int Méx 2007; 23(5): 464-470.
[8] Ribalta J. Mecanismos fisiopatológicos de la esteatosis microvesicular (hígado graso agudo del embarazo). Medwave, Edición Enero 2003. IV Curso Bienal Internacional de Ciencias en Gastroenterología Esteatohepatitis, Chile. 8-09-2003,
[9] Wenk RE, Gebhardt FC, Bhagavan BS, Lustgarten JA, McCarthy EF. Tetracycline-associated fatty liver of pregnancy, including possible pregnancy risk after chronic dermatologic use of tetracycline. J Reprod Med 1981; 26:135-141.
[10]. Fondo Nacional de Ciencia, Tecnología e Innovación (FONACIT). Norma para utilización de animales en investigación. En: Código de Bioética y Bioseguridad. Capítulo 3. Tercera edición. Caracas, Venezuela, pp 33-35. 2009.
[11] Dufour RD, Lott, JD, Nolte, FS,. Gretch, DR, Koff, RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance Characteristics of Laboratory Tests. Clin Chem 2000; 46: 2027-2049.
[12] Patton JG, Dinh DM, Mao SJ. Phospholipid enhances triglyceride quantitation using an enzyme kit methods. Clin Chim Acta 1982; 118(1): 125-128.
[13] Bradford MA. A rapid and sensitive method for the quantities of microgram of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248-254.
[14] Wallin B, Rosengren B, Shertzer H, Camejo G. Lipoprotein oxidation and measurement of thiobarbituric acid reacting substances formation in a single microtiter plate: Its use for evaluation of antioxidants. Anal Biochem 1993; 208, 10-15.
[15] Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-358.
[16] Shen C, Meng Q, Schmelzer E, Bader A. Gel entrapment culture of rat hepatocytes for investigation of tetracycline-induced toxicity. Toxicol Appl Pharmacol 2009; 238(2): 178-187.
[17] Amacher DE, Martin BA. Tetracycline-induced steatosis in primary canine hepatocyte cultures. Fundam Appl Toxicol 1997; 40: 256-263.
[18] Alam S, Noor-E-Alam SM, Chowdhury ZR, Alam M, Kabir J. Nonalcoholic steatohepatitis in nonalcoholic fatty liver disease patients of Bangladesh. World J Hepatol 2013; 5(5): 281-287.
[19] Giannini EG, Testa R, Savarino V. Liver enzyme alteration: A guide for clinicians. Can Med Associat J 2005; 172(3): 367-379.
[20] Larreal YL, Andrade EL, Cuevas YE, Mendoza AS, Montiel MV, Levy AC, Valero NJ. Pruebas de funcionalismo hepático en pacientes con infección viral aguda. Acta Bioquím Clín Latinoam 2012; 46(1): 38-46.
[21] Yang SH, Chang SY, Tu Y, Lawson GW, Martin O. Bergo MO, Fong LG, Young SG. Severe hepatocellular disease in mice lacking one or both CaaX prenyltransferases. J Lipid Res 2012; 53(1): 77-86.
[22] Zhang YKJ, Yeager RL, Tanaka Y, Klaassen CD. Enhanced expression of Nrf2 in mice attenuates the fatty liver produced by a methionine- and choline-deficient diet. Toxicol App Pharmacol 2010; 245(3): 326-334.
[23] Halliwell, B. y Gutteridge, J. (2015). Free radicals in biology and medicine. 5ta Ed. Editorial Oxford Universsity Press. pp 140-150.
[24] Sordillo LM, O’Boyle N, Gandy JC, Corl CM, Hamilton E. Shifts in Thioredoxin reductase activity and oxidant status in mononuclear cells obtained from transition dairy cattle. J Dairy Sci 2007; 90: 1186-1192.
[25] Lau JKC, Zhang X, Yun J. Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J Pathol 2017; 241(1): 36-44.
Published
How to Cite
Issue
Section
Gaceta de Ciencias Veterinarias se apega al modelo Open Access, por ello no se exige suscripción, registro o tarifa de acceso a los usuarios o instituciones. Los usuarios pueden leer, descargar, copiar, distribuir, imprimir y compartir los textos completos inmediatamente después de publicados, se exige no hacer uso comercial de las publicaciones. Para la reproducción parcial o total de los trabajos o contenidos publicados, se exige reconocer los derechos intelectuales de los autores y además, hacer referencia a esta revista. La publicación de artículos se hace sin cargo para los autores. Los trabajos pueden consultarse y descargarse libremente, y de manera gratuita, en extenso en versión digital, desde su enlace Web institucional. Los textos publicados son propiedad intelectual de sus autores. Las ideas, opiniones y conceptos expuestos en los trabajos publicados en la revista representan la opinión de sus autores, por lo tanto, son estos los responsables exclusivos de los mismos.


