Presence of Glutathione-S-transferase enzime in bovine luteal body.
Abstract
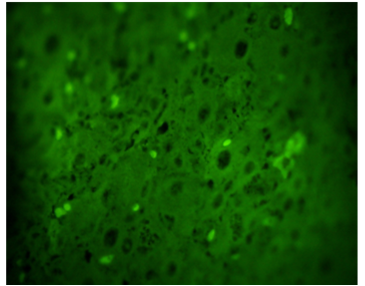
The objective of the present investigation was to identify the presence of enzyme Glutathion-S-transferase with direct immunoflorescence technique in mature luteal bodies (LC) and in regression of dairy cattle. For this purpose, 133 CL of Holstein cows, non-pregnant adults, and two or more lactations were sampled from a commercial farm located in the municipality of Jiménez, Lara State, Venezuela, slaughtered in two industrial slaughterhouses, once obtained the ovaries were transferred in a phosphate buffered saline solution at a temperature of 4 to 8 °C to "Dr Haity Moussatche" Research Unit Laboratory of the Veterinary Sciences Dean of UCLA (DVS-UCLA ) To be assayed, 5 μm slices were subsequently taken where the presence of GST enzyme was determined in each sample and incubated with diluted GST antibody. Descriptive statistics were applied for results analysis. It was observed that in the mature LC, 71.25% had a high content of fluorescent granules (presence of the enzyme) while the remaining 28.75% presented moderate content. On the other hand the LC in regression showed little amount of fluorescent green granules indicating little expression of the enzyme.
Downloads
References
[2] Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD: Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol 2013; 1: 304-312.
[3] Minegishi K, Tanaka M, Nishimura O, Taniyaki S, Miyakoshi K, Ishimoto H, Yoshimura Y. Reactive oxygen species mediate leukocyte-endothelium interactions in prostaglandin F2α- induced luteolysis in rats. Am J Physiol Endocrinol Metab 2002; 283: 1308-1315.
[4] Sawada M, Carlson J. Studies on the mechanism controlling generation of superoxide radical in luteinized rat ovaries during regression. Endocrinology 1994; 135 (4): 1645-1250.
[5] Miller J, Brzezinska-Slebodzinska E, Madsen F. Oxidative stress, antioxidants and animal function. J Dairy Sci 1993; 76(9): 2812-2823.
[6] Musicki B, Aten R, Behrman H. Inhibition of protein synthesis and hormone-sensitive steroidogenesis in response to hydrogen peroxide in rat luteal cells. Endocrinology 1994; 134 (2): 588-595.
[7] Sugino N, Nakamura Y, Takeda O, Ishimatsu M, Kato H. Changes in activities of superoxide dismutase and lipid peroxide in corpus luteum during pregnancy in rats. J Reprod Fertil 1993; 97: 347-351.
[8] Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002; 82: 47-95.
[9] Sugino N, Kato H. The role of ischemia-reperfusion injuries in generation reactive oxygen species during luteolysis. Adv Contracep Deliv Syst 1994; 10: 95-106.
[10] Rapoport R, Sklan D, Wolfenson D, Shaham-Albalancy A, Hanukoglu I. Antioxidant capacity is correlated with steroidogenic status of the corpus luteum during the bovine estrous cycle. Biochim. Biophys Acta 1998; 1380: 133-140.
[11] Al-Gubory KH, Garrel C, Faure P, Sugino N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reproduc BioMed Online 2012; 25: 551-560.
[12] Udomsinprasert R, Pongjaroenkit S, Wongsantichon J, Oakley AJ, Prapanthadara LA, Wilce MC, Ketterman AJ. Identification, characterization and structure of a new Delta class glutathione transferase isoenzyme. Biochem J 2005; 388: 763-771.
[13] Allocati N, Federici L, Masulli M, Di Ilio C. Glutathione transferases in bacteria. FEBS J 2009; 276 (1): 58-75.
[14] Sheehan D, Meade G, Foley V, Dowd C. Structure, function and evolution of Glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 2001; 360: 1-16.
[15] Al-Gubory K, Bolifraud P, Germain G, Nicole A, Ceballos-Picot I. Antioxidant enzymatic defence system in sheep corpus luteum throughout pregnancy. Reprod Res 2004; 128 (6): 767-774.
[16] Ito M, Muraki M, Takahashi F, Imai M, Tsukui T, Yamakawa N, et al. Glutathione S-transferase theta 1 expressed in granulosa cells as a biomarker for oocyte quality in age-related infertility. Fertil Steril 2008; 90 (4): 1026-1035.
[17] Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R et al. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod 2003; 9: 639-643.
[18] Suzuki K, Tatsumi H, Satoh S, Senda T, Nakata T, Fujii J, Taniguchi N. Manganese-superoxide dismutase in endothelial cells: Localization and mechanism of induction. Am J Physiol Heart Circ Physiol 1993; 265: 1173-1178.
[19] Rueda B, Tilly K, Hansen T, Hoyer P, Tilly J. Expression of superoxide dismutase, catalase and glutathione peroxidase in the bovine corpus luteum: evidence supporting a role for oxidative stress in luteolysis. Endocrine 1995; 3: 227-232.
[20] Zhong W, Yan T, Lim R, Oberley L. Expression of Superoxide Dismutase, Catalase and Glutathione peroxidase in Glioma cells. Free Rad. Biol. Med 1999; 27(12): 1334-1345.
[21] Márquez YC, Márquez A; Fuentes M; Salas Y; López–Ortega A. Estado oxidativo de cuerpos lúteos maduros y regresivos en bovinos. Rev. Vet 2011. 22: 1, 25-31.
[22] Pullman N. Condition scoring in Fulani cattle. Tropical Ani. Health 1978; 10: 118-120.
[23] Márquez Y. Determinación de los parámetros de peroxidación lipídica en células lutéales de hembras bovinas lecheras. Trabajo de Ascenso. Decanato de Ciencias Veterinarias. UCLA. Venezuela 2001.
[24] Edna P. Procesamiento de Tejidos: Deshidratación, Aclaramiento, e Infiltración. Métodos Histotecnológicos. Registro de Patología de los Estados Unidos de América (ARP) y por el Instituto de Patología de las Fuerzas Armadas de los Estados Unidos de América (AFIP). 1995; pp. 31-33.
[25] Bob M. Orientación del Espécimen. Métodos Histotecnológicos. Registro de Patología de los Estados Unidos de América (ARP) y por el Instituto de Patología de las Fuerzas Armadas de los Estados Unidos de América (AFIP). 1995; pp. 35-46.
[26] Zangar RC, Davydov DR, Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol 2004; 199, 316-331.
[27] Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem. Cell Biol 2007; 39, 44-84.
[28] Arianmanesh M, McIntosh R, Lea RG, Fowler PA, Al-Gubory KH. Ovine corpus luteum proteins, with functions including oxidative stress and lipid metabolism, show complex alterations during implantation. J Endocrinol 2011; 210, 47-58.
[29] Nakamura T, Ishigami T, Makino N, Sakamoto K. The downregulation of glutathione peroxidase causes bovine luteal cell apoptosis during structural luteolysis. J Biochem 2001; 129, 937-942.
Published
How to Cite
Issue
Section
Gaceta de Ciencias Veterinarias se apega al modelo Open Access, por ello no se exige suscripción, registro o tarifa de acceso a los usuarios o instituciones. Los usuarios pueden leer, descargar, copiar, distribuir, imprimir y compartir los textos completos inmediatamente después de publicados, se exige no hacer uso comercial de las publicaciones. Para la reproducción parcial o total de los trabajos o contenidos publicados, se exige reconocer los derechos intelectuales de los autores y además, hacer referencia a esta revista. La publicación de artículos se hace sin cargo para los autores. Los trabajos pueden consultarse y descargarse libremente, y de manera gratuita, en extenso en versión digital, desde su enlace Web institucional. Los textos publicados son propiedad intelectual de sus autores. Las ideas, opiniones y conceptos expuestos en los trabajos publicados en la revista representan la opinión de sus autores, por lo tanto, son estos los responsables exclusivos de los mismos.


